Week 6: My last week!
Day 25 and 26:
LC3B was identified successfully in all of the lanes: The brightness of the LC3B in each of the lanes:
In lane 2: 2620, In lane 3: 4130, In lane 364, In lane 5: 1390, In lane 6: 3690.
Result of my western blot:
In lane 2: 2620, In lane 3: 4130, In lane 364, In lane 5: 1390, In lane 6: 3690.
Plating the cells for this week:
Immunofluorescence: The plates
I split the cells that I had incubated in the flask in order to put them in the immunofluoresnce plates. Seeing as there were 24 wells in total, I wanted to plate enough cells for 28 wells to make up for pipetting error. 30, 000 cells per plate were required therefore 30, 000 x 28 = 840, 000. I found that after splitting the cells i had 997, 600 cells/ml. Thus, 840, 000 / 997, 600 = 842ul required. The amount of media required: 750ul x 28 = 21ml media.
I had to sterilise coverslips to attach the cells to coverslips for subsequent confocal #microscopy. After sterilising the coverslips with 70% ethanol, I carefully (without breaking them!) placed them back into the wells. I then plated 750ul in each of the wells and incubated them for later OGD experiments the following day.
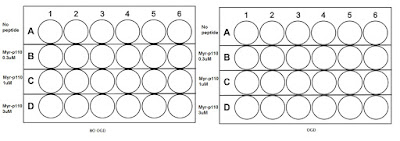
OGD experiments and varying concentrations of TAT-p110 and Myr-p110: The plates
500ul per well is needed and 25, 000 wells are needed in each well there are 96 wells in total here and therefore I made enough for 100 wells to make up for pipetting error.
25, 000 x 100 = 2, 500, 000. 2, 500, 000/997, 600 = 2.5ml cells. 500ul x 100 = 50ml media. I placed 500ul in each well in the 24 well plate and incubated the plate overnight.
The next day: OGD and treating the plates with TAT, Myr-p110 and TAT-p110.
I obtained the OGD plates and aspirated out the old media to then aliquot 750ul aCSF in the immunofluorescence OGD plate and 500ul aCSF in the varying treatment concentration plate.
 |
An image of the coverslip plate with aCSF in (clear liquid) before it entered the hypoxia chamber
|
After an hour and a half of OGD, the relevant plates were retrieved and treated with the peptides.
The volumes of drug administered for the plates:
Calculation of the
volumes of each of the diluted peptides required to make up final concentration
of 1μM 750μl media:
Use the equation V = n/C
For TAT and Myr-p110 (Both 10mM stock solutions):
To make 1 μM final:
Use 1mM dilution tube, ie. 1/10. 1mM is the same as 1000 μM.
Therefore: 1 μM/1000 μM = 1x10-3.
1x10-3 x 750 μl = 0.75 μl/750 μl media in ONE WELL.
For 10 wells: 0.75 μl / 750 μl x 10 = 7.5 μl/7.5ml media
Therefore: 1 μM/1000 μM = 1x10-3.
1x10-3 x 750 μl = 0.75 μl/750 μl media in ONE WELL.
For 10 wells: 0.75 μl / 750 μl x 10 = 7.5 μl/7.5ml media
For TAT-p110 (4mM Stock solution):
To make 1 μM final:
Use 0.4mM dilution tube, ie. 1/10. 0.4mM is the same as 400 μM.
Therefore: 1 μM/400 μM = 2.5x10-3.
2.5x10-3 x 750 μl = 1.875 μl/750 μl media in ONE WELL. For 10 wells: 1.25 μl / 750 μl x 10 = 18.75 μl/7.5ml media
Therefore: 1 μM/400 μM = 2.5x10-3.
2.5x10-3 x 750 μl = 1.875 μl/750 μl media in ONE WELL. For 10 wells: 1.25 μl / 750 μl x 10 = 18.75 μl/7.5ml media
- I left the plates with varying concentration of drug in the incubator for assays the next day.
Fixing the cells on the coverslips plate for subsequent immunoflouresence:
I had to aspirate off the media containing the drugs which treated the cells and added 750ul of PFA, a fixing reagent, into each of the plates for 10 minutes at room temperature, then washed the wells with PBS. It was necessary that the cells were slightly permeablised to allow the primary and secondary flourenscent antibodies to enter. The permeablisation buffer was made by adding 500ul 0.1% Triton to 50ml PBS. I aliquoted 750ul to each of the wells and incubated the cells for 5 minutes, then washed again with PBS.
Similarly to western blotting, a blocking mixture needed to be made for this to ensure that nonspecific binding of the antibodies onto the cells was minimised as much as possible. I weighed out 0.125g BSA and mixed it in 50ml PBS and placed 750ul in each of the wells, incubating it at room temperature for 30minutes.
Similarly to western blotting, a blocking mixture needed to be made for this to ensure that nonspecific binding of the antibodies onto the cells was minimised as much as possible. I weighed out 0.125g BSA and mixed it in 50ml PBS and placed 750ul in each of the wells, incubating it at room temperature for 30minutes.
Primary antibody incubation:
The primary antibodies I used were for TOMM20 and Drp1.
I had to arrange the coverslips in such a way that they could be covered in a mixture of the primary antibody in blocking solution. Parafilm is a special film which I used to arrange and incubated the coverslips in.
I placed a 30ul drop of the primary antibody mixture on the parafilm for each of the coverslips, covered the plate in foil and incubated in the fridge overnight...






Comments
Post a Comment