Week 4: Inhibiting mitochondrial fission using p110 peptide varients in an attempt to prolong cell survival during neonatal hypoxic ischaemia
The theory behind
the experiment:
- There is an increase in mitochondrial mitophagy during
neonatal hypoxic ischaemia. The cell becomes stressed as a result of the
reduced oxygen in the environment. Reactive oxygen species (ROS) being formed
after electrons react with molecular oxygen in the electron transport chain.
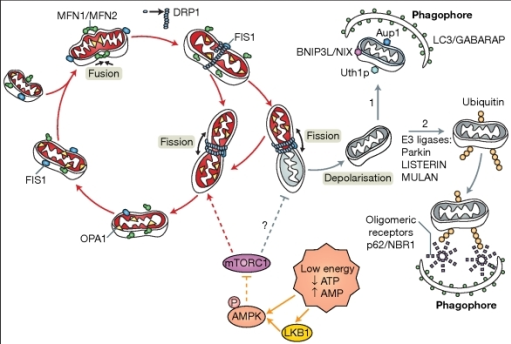
The mitochondria which have become damaged will undergo the process of mitochondrial
fission, mediated by the proteins Drp1, MFF, MID49/51 and Fis1. The
mitochondria which have accumulated damage will have their damaged features
taken to just one area in the mitochondrion. Once fission has occurred and one
mitochondrion has become two, the mitochondrion which had accumulated its
damage will undergo mitochondrial mitophagy.
- As a result, less ATP is available for the cell but more ROS is produced. In addition, the mitochondrial membrane also becomes leaky releasing apoptosis stimulating factors and cytochrome c, therefore stimulating apoptosis and exaggerating the effects observed during neonatal hypoxic ischaemia.
- The healthy mitochondria may undergo the process of fusion using the mitochondrial fusion protein OPA1, forming one larger mitochondrion and theoretically producing more ATP. Unfortunately, during neonatal hypoxic ischaemia, OPA1 also becomes damaged and more ATP cannot be produced by mitochondrial fusion, thus, targeting fission may be the only way to replenish ATP during neonatal hypoxic ischaemia.
- As a result, less ATP is available for the cell but more ROS is produced. In addition, the mitochondrial membrane also becomes leaky releasing apoptosis stimulating factors and cytochrome c, therefore stimulating apoptosis and exaggerating the effects observed during neonatal hypoxic ischaemia.
- The healthy mitochondria may undergo the process of fusion using the mitochondrial fusion protein OPA1, forming one larger mitochondrion and theoretically producing more ATP. Unfortunately, during neonatal hypoxic ischaemia, OPA1 also becomes damaged and more ATP cannot be produced by mitochondrial fusion, thus, targeting fission may be the only way to replenish ATP during neonatal hypoxic ischaemia.
The hypothesis:To be able to inhibit mitochondrial mitophagy transiently,
before fission occurs, thus increasing ATP levels in the cell. This will
promote ATP production despite the fact that ROS has been produced. By
inhibiting mitochondrial fission, we may be able to prevent the increase in
mitophagy and thus keep ATP levels higher for longer.
The fundamentals
of p110 and how it relates to mitochondrial fission:Amino acid sequence: DLLPRGT. When fission needs to happen,
Drp1 is in the cytosol and is phosphorylated at position 637. Drp1 is recruited
to the mitochondria and binds to its complementary mitochondrial receptor,
Fis1. Once this happens, Drp1 gets phosphorylated on position 616. The function
of p110 is such that it inhibits the interaction between Fis1 and Drp1 by competitive
inhibition upon Fis1 binding. Thus, Drp1 cannot bind and cannot be
phosphorylated, ultimately inhibiting the process of mitochondrial fission.
There are 3 different related peptides which we decided to
test: TAT, Myristilated-p110 and TAT-p110. TAT had no peptide associated with
it and thus could be seen as a control. P110 alone simply enters the cell
through a channel protein through the membrane of the cell. TAT-p110 enters the
cell by being loaded into a vesicle and entering the cell by endocytosis. Myr-p110
enters the cell by simple diffusion thus indicating that it should have an
easier time transporting the peptide into the cell.
Preparation of the peptide: The peptides needed to be
prepared to make up a final stock concentration of 10mM. TAT and Myr-p110 were
successfully dissolved in PBS. When it came to dissolving TAT-p110, however,
the solution still appeared cloudy in the PBS, thus it also needed to be
dissolved in 20% DMSO making a final concentration of 4mM.
We split and plated the cells ready for the next day for
cytotoxicity tests…



Comments
Post a Comment