Week 3
Day 11: Splitting cells, my first mistake and calculating protein concentrations
I managed to split the C17 cells on my own and unassisted from two previously split flasks. The aim was to keep the spare cells for oxygen glucose deprivation experiments and transfections with mCherry.
What is mCherry?
mCherry is a special flurophore which is used in molecular biology techinques as a tracer to track the localisation of molecules or organelles. In this case, we wanted to use it to view mitochondria. Unlike Keima or JC1 which I previously had experience with, mCherry would simply flurorece bright red once it was localised to mitochondria in the cells.
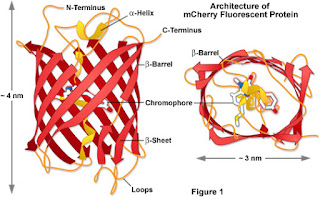
The structure of mCherry:
The structure of mCherry is such
that it is a beta barrel motif with a fluorophore which is attached on the gap
of the beta barrel. In addition there is also an alpha helix which runs down
the beta barrel stabilising the chromophore in the centre.
The theory behind using Cherry with relation to our
experiment was that if we had OGD samples compared with control samples, by
transfecting the plates with mCherry, we would expect to see a difference in
the extent if the fluorescence in the chromophore’s fluorescence.
I made my first mistake! It had its consequences…
I was required to split my cells in the flask we kept from
last week into two separate flasks and keep the spare volume of cells and media
for oxygen glucose experiments and transfection for the mCherry experiments. I accidently
disposed of them into a beaker of detergent which meant that we had to postpone
our experiments for next week.
Luckily, there were pre prepared cell lysates from a different experiment completed on C17 cells made of controls and treated with oxygen/glucose deprivation as well as a mitochondrial fission inhibitor. I was required to calculate the protein concentration of all 24 cell lysate samples so I could perform western blots later on them.
The theory behind the experiment:
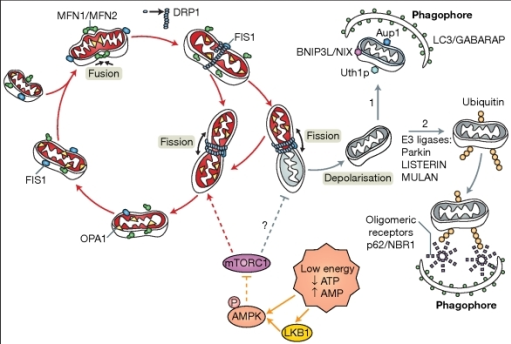
 |
| The process of fission with mitochondria (Hagberg et al., 2014) |
During cellular stress, such as undergoing hypoxic ischaemia,
mitochondria attempt to compensate for the loss of ATP by undergoing a process
called mitochondrial fission and then grow to produce more ATP. In the brain of
a developing foetus, the mitochondria form and they undergo contestant cycles
of fission and fusion to meet the energy demands of the cell. If a
mitochondrion has accumulated damage in one area of the organelle, such as
reactive oxygen species which can negatively affect mitochondrial proteins and
DNA, then the damage becomes polarised on one side of the organelle and
undergoes fission.
The resulting two new mitochondria will have two
destinations: the mitochondria with the accumulated damage will go through
mitophagy whilst the new healthy mitochondrion will grow and continue to
produce ATP. However, during perinatal hypoxic ischaemia, the damage
accumulates in mitochondria excessively, resulting in excessive fission and mitochondria
undergoing mitophagy before they have had the opportunity to grow to their mature
state. During hypoxic ischaemic brain injury, there is a 6-hour window to raise
ATP levels in the cells to the optimum level and restore normal metabolism. If fission
was inhibited, the mitochondria would have a chance to grow and compensate for
the loss of ATP properly despite its own accumulated damage.

Comments
Post a Comment