Week 1
Hello! My name is Afra, I am a third year undergraduate student studying Biochemistry at King's College London. During this summer, I have been lucky enough to receive a grant from the Biochemical society and undergo a research studentship in identifying the components of mitophagy in relation to perinatal brain injury.
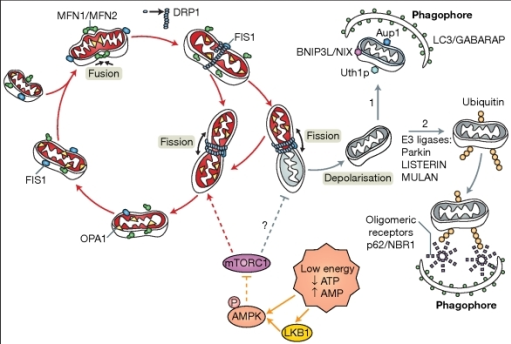
Hypoxic ischaemic brain injury is as a result of a lack of oxygen getting to the brain from the time of birth. Perinatal brain injury affects 1-3 in 1000 new born babies and the consequences of its occurrence may be fatal. At the moment, the only treatment available for neonatal hypoxic ischaemic brain injury is placing the infants in hypothermia conditions. Despite the fact that this has appeared to be effective, it only cures approximately 1-7 babies. Thus, it is important to search for other methods along with hypothermia to treat this condition. It has been implicated that excessive mitochondrial mitophagy may be a component of the later brain injury.
For the next 6 weeks, I will be working in a lab attempting to identify the components of mitochondrial mitophagy and whether they are truly implicated in neonatal hypoxic iscaemic brain injury
Western blotting
Day one: my first western blot!
Mice brains have been taken and sampled using the vannucci method, which is where the carotid artery supplying the ipsilateral side of the brain is ligated, thus stopping the blood flow to that region. This, combined with being exposed to 10% oxygen will eventually mean that brain tissue on the ipsilateral side of the brain disappears because of progressive and substantial cell death (apoptosis). The neuronal tissue on the contralateral side of the brain is maintained despite its exposure to the 10% oxygen, because the carotid artery supplying it is not blocked. The neonatal mice brains can be extracted at different times, eg. 0 hrs, 2hrs and 24 hrs for both the cytosol and the mitochondria. Unlike human neonate, mice brains continue to develop even after birth and the type of mice used were term equivalent (p9)
Western blotting – separating the protein in the cytosol of
the cell and from mitochondria to identify components of the mitophagy pathway
eg. Parkin (52 kDa), PINK1 (63 kDa), ubiquitin (8.5 kDa).Mice brains have been taken and sampled using the vannucci method, which is where the carotid artery supplying the ipsilateral side of the brain is ligated, thus stopping the blood flow to that region. This, combined with being exposed to 10% oxygen will eventually mean that brain tissue on the ipsilateral side of the brain disappears because of progressive and substantial cell death (apoptosis). The neuronal tissue on the contralateral side of the brain is maintained despite its exposure to the 10% oxygen, because the carotid artery supplying it is not blocked. The neonatal mice brains can be extracted at different times, eg. 0 hrs, 2hrs and 24 hrs for both the cytosol and the mitochondria. Unlike human neonate, mice brains continue to develop even after birth and the type of mice used were term equivalent (p9)
The method
conducted:
Samples of p9 neonatal mice brains were extracted from the contralateral and the ipsilateral sides.
- Once the samples have been prepared, they are all placed on a heat block for 5 minutes at 90 degrees, this is with β-mercaptoethanol which helps to break disulfide bonds to denature the mitochondrial and cytosolic protein samples.
Samples of p9 neonatal mice brains were extracted from the contralateral and the ipsilateral sides.
- Once the samples have been prepared, they are all placed on a heat block for 5 minutes at 90 degrees, this is with β-mercaptoethanol which helps to break disulfide bonds to denature the mitochondrial and cytosolic protein samples.
The gel and the lanes:
Once the gels were prepared, it was required that
they underwent western blotting, so the protein that ran on the gel could be
visualised by using a special membrane, PVDF.
The western blotting procedure proved to be quite difficult at first, as there was a lot of handling of wet sponges and western blotting apparatus. After some practice, however, I eventually got the hang of it! It was important that we kept the western blot apparatus cool with ice while it was running, and we needed to wait two hours to allow the maximum possible amount of protein to be transferred. The PVDF membrane was cut in half and this is the PVDF membrane with the protein ladder marker transferred onto it. We used milk to block the membrane. this process is important in order to increase specificity of the primary antibodies when locating the Parkin protein. We left the membrane in the milk for approximately half an hour and washed it with buffer TBST.
Once it was washed, we needed to prepare primary antibody solutions that were specific for Parkin. We used the same antibody from two different companies. The membranes needed to be immersed in a mixture of TBST for 24 hours in a cold room of 4°C.
The western blotting procedure proved to be quite difficult at first, as there was a lot of handling of wet sponges and western blotting apparatus. After some practice, however, I eventually got the hang of it! It was important that we kept the western blot apparatus cool with ice while it was running, and we needed to wait two hours to allow the maximum possible amount of protein to be transferred. The PVDF membrane was cut in half and this is the PVDF membrane with the protein ladder marker transferred onto it. We used milk to block the membrane. this process is important in order to increase specificity of the primary antibodies when locating the Parkin protein. We left the membrane in the milk for approximately half an hour and washed it with buffer TBST.
Once it was washed, we needed to prepare primary antibody solutions that were specific for Parkin. We used the same antibody from two different companies. The membranes needed to be immersed in a mixture of TBST for 24 hours in a cold room of 4°C.




Comments
Post a Comment