Day 27 and 28:
Assays, secondary antibody incubation and splitting cells:
The assays:
I ran assays for all of the OGD plates for Hoechst, MTT and LDH and the results were as follows:
The slides were labelled with their relevant characteristic and the cloverslips were placed on top. The coverslips were secured in place with nailvarnish to the microscope slides and left to dry in the dark.
Assays, secondary antibody incubation and splitting cells:
The assays:
I ran assays for all of the OGD plates for Hoechst, MTT and LDH and the results were as follows:
Secondary antibody incubation for immunofluorescence:
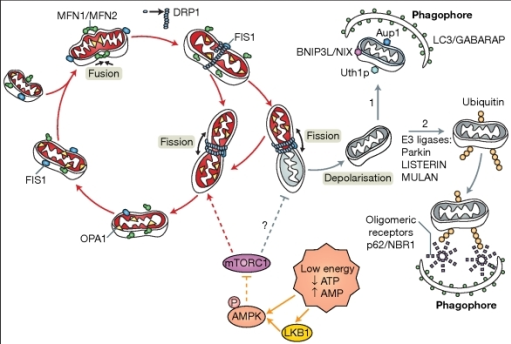
I had to incubate the coverslips with secondary antibodies against the TOMM20 (which would be an indicator of mitochondrial morphology and presence) and Drp1 (which would be an indicator of fission. TOMM 20 were going to appear red whilst Drp1 was going to appear green on the confocal microscope. I incubated the coverslips in darkness for the rest of the day. At the end of the day, I mounted the coverslips onto slides which contained DAPI which would mark the nucleus and this would appear blue on the confocal microscope.
Mounting the coverslips onto the slides:
The slides were labelled with their relevant characteristic and the cloverslips were placed on top. The coverslips were secured in place with nailvarnish to the microscope slides and left to dry in the dark.
Conducting the OGD experiment:
After judging from the previous OGD experiment, we decided to plate new cells for a new OGD experiment which would look into varying the concentration of TAT and TAT-p110 on the cells to assess whether changing the concentration of the drug would improve cell survival. I plated the cells as follows:





Comments
Post a Comment