Day 19: oxygen glucose deprivation experiment on C17.2 cells
- I labelledup the plates with either Control, 1.5 hr OGD or 3
hr OGD.
- Obtain the 1.5hr plate and the 3hr plate and aspirate the media from them. Wash each well out with 500µl of PBS and aspirate that out as well to ensure that there is no more media left in the wells.
- place 500µl of the artificial CSF in both the 1.5 and 3 hour plates.
- I took both plates and place them into separate hypoxia chambers. Start with the 1.5hr plate. Flood the chamber with nitrogen for one minute and clasp the tubes shut when complete.
- Place the chamber back into the incubator and set the timer for 90 minutes.
- Obtain the 3 hour plate and place it into the other hypoxia chamber. Also flood this chamber with nitrogen for one minute, clasp the clips shut and place it into the incubator. Set the timer for 3 hours.
- while the oxygen glucose deprivation is happening, prepare the peptides as follows:
- Obtain the 1.5hr plate and the 3hr plate and aspirate the media from them. Wash each well out with 500µl of PBS and aspirate that out as well to ensure that there is no more media left in the wells.
- place 500µl of the artificial CSF in both the 1.5 and 3 hour plates.
- I took both plates and place them into separate hypoxia chambers. Start with the 1.5hr plate. Flood the chamber with nitrogen for one minute and clasp the tubes shut when complete.
- Place the chamber back into the incubator and set the timer for 90 minutes.
- Obtain the 3 hour plate and place it into the other hypoxia chamber. Also flood this chamber with nitrogen for one minute, clasp the clips shut and place it into the incubator. Set the timer for 3 hours.
- while the oxygen glucose deprivation is happening, prepare the peptides as follows:
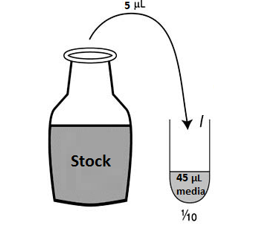
Dilution of the
peptides for OGD experiments:
1μM is needed of each of the peptides for these experiments,
therefore a 1/10 dilution of the stock of the peptide is required in epindorph
tubes.
- The TAT and Myr-p110 were both 10mM stock solutions whilst
TAT-p110 was 4mM stock solution as it would not dissolve properly previously
Calculation of the
volumes of each of the diluted peptides required to make up final concentration
1μM in each well for 500μl media:
Use the equation V = n/C
NOTE: there are 18 wells in total for each peptide. 6 wells
per plate for each peptide per plate and there are 3 plates. Therefore 6x3 = 18
wells. We need to make enough solution of each peptide in for all of the total
plates. Due to pipetting error, scale it up to 20 wells.
For TAT and Myr-p110 (Both 10mM stock solutions):
To make 1 μM final: Use 1mM dilution tube, ie. 1/10. 1mM is the same as 1000 μM.
Therefore: 1 μM/1000 μM = 1x10-3.
1x10-3 x 500 μl = 0.5 μl/500 μl media in ONE WELL.
For 20 wells: 0.5 μl / 500 μl x 20 = 10 μl/10ml media
To make 1 μM final: Use 1mM dilution tube, ie. 1/10. 1mM is the same as 1000 μM.
Therefore: 1 μM/1000 μM = 1x10-3.
1x10-3 x 500 μl = 0.5 μl/500 μl media in ONE WELL.
For 20 wells: 0.5 μl / 500 μl x 20 = 10 μl/10ml media
After 90 minutes:
- I took out the hypoxia chamber that had the 1.5 hr plate in.
Aspirate out all of the artificial CSF.
- I placed 500 μl media in the control row, 500 μl of 1 μM TAT in the TAT row, 500 μl Myr-p110 in the Myr-p110 row and 500 μl 1 μM TAT-p110 into the TAT-p110 row.
- I placed 500 μl media in the control row, 500 μl of 1 μM TAT in the TAT row, 500 μl Myr-p110 in the Myr-p110 row and 500 μl 1 μM TAT-p110 into the TAT-p110 row.
For the control plate:
- I aspirated out the old media,
- Place 500 μl media in the control row, 500 μl of 1 μM TAT in the TAT row, 500 μl Myr-p110 in the Myr-p110 row and 500 μl 1 μM TAT-p110 into the TAT-p110 row.
- I aspirated out the old media,
- Place 500 μl media in the control row, 500 μl of 1 μM TAT in the TAT row, 500 μl Myr-p110 in the Myr-p110 row and 500 μl 1 μM TAT-p110 into the TAT-p110 row.
After 3 hours:
- I took out the hypoxia chamber that had the 3 hr plate in. Aspirate out all of the artificial CSF.
- I placed 500 μl media in the control row, 500 μl of 1 μM TAT in the TAT row, 500 μl Myr-p110 in the Myr-p110 row and 500 μl 1 μM TAT-p110 into the TAT-p110 row.
- I took out the hypoxia chamber that had the 3 hr plate in. Aspirate out all of the artificial CSF.
- I placed 500 μl media in the control row, 500 μl of 1 μM TAT in the TAT row, 500 μl Myr-p110 in the Myr-p110 row and 500 μl 1 μM TAT-p110 into the TAT-p110 row.
- Leave for 24 hours and leave the plates for observation
the next day..

Comments
Post a Comment